how many valence electrons does nitrogen have
The electronic configuration of Bromine is 1s22s22p63s23p64s23d104p5 and the valence electrons are in the 4s and 4p orbitals giving Bromine 7 valence electrons. Nitrogen makes up DNA both in the form of nitrogen bases and in neurotransmitters.

Periodic Table With Valence Electrons Labeled 7 Hd Images
From the above electron configuration of nitrogenwe see that nitrogen has 5 valence electrons in its valence shell.
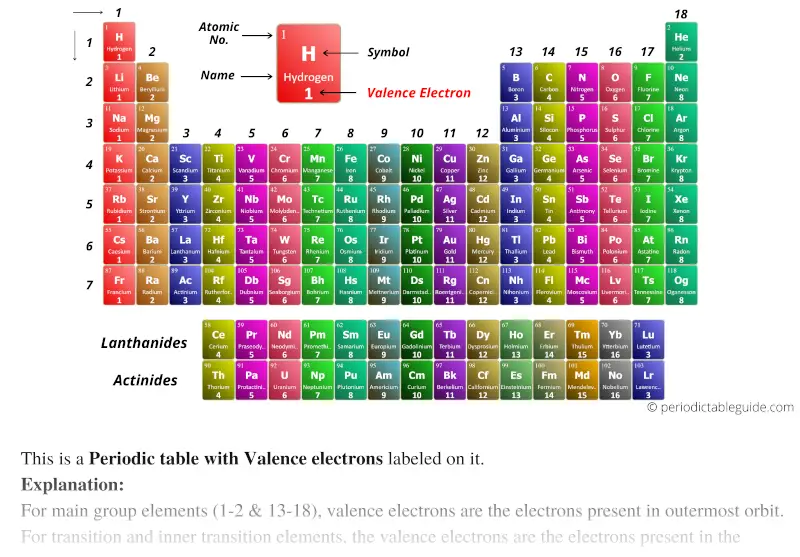
. You can determine the number of valence electrons an element has by looking at the Periodic Table. RowsLeft to right on the periodic table. How Many Valence Electrons Does Nitrogen Have. The last shell of nitrogen has five electrons so the valence electrons of nitrogen have five.
How many valence electrons does Nitrogen N have. Nitrogen has 5 valence electrons since its electron construction is. 5 valence electrons Nitrogen has a total of 5 valence electrons. In the diagram above we show nitrogen making 3 bonds.
New questions in Chemistry. It can have either 3 or 5 valence electrons as it can bond in the 2p and 2s orbitals outside. Nitrogen is the 7th element so the end of 7 electrons 2 walk to the very first shell and 5 come the second. Thus the total number of valence electrons present in nitrogen atoms is five.
At normal temperature and pressure two atoms of nitrogen bind togetherto type colorless and odorless dinitrogen N2 gas. Nitrogen is in column 15 also called Group 5A and therefore Nitrogen has 5 valence electrons. Look at the number above the groups on the periodic table. This article discusses in detail the valence electrons of nitrogen.
It needs to make 3 bonds to get an octet. How Many Valence Electrons Does Nitrogen HaveNumber of Valence Electrons inOffor NitrogenDoes nitrogen have 3 or 5 valence electronsHow many valenc. We have two electrons in orbital s. Thus nitrogen has five valence electrons.
Nitrogen has 5 valence electrons because its electron configuration is 1 s 2 2 s 2 2 p 3. There is a quick way of identifying the number of valence electrons - it is the same as the Group number not for d-block elements though. How many valence electrons does Magnesium Mg have. How many valence electrons does nitrogen Nitrogen achemical facet with the prize Si and atomic number 14 is a colorless liquidgas or solid.
1 See answer jayddani9562 is waiting for your help. To determine valence electrons add the outermost and orbitals. It has 5 valence electrons. You can also look at the electron configuration of Nitrogen to determine the number of valence electrons.
119 rows We have shown the Valence Electrons of the elements for which reliable data. Nitrogen has either 3 or 5 valence electrons and is on the periodic table at the top of Group 15. So there are 5 valence electrons of nitrogen. Also why does BR have 7 valence electrons.
Nitrogen being a non-metal has five electrons in its outermost shell and requires only three electrons to complete its octet and acquire stable electronic configuration of the nearest noble gas So nitrogen has a strong tendency to gain three electrons from the other electron donating species and thus it acts as a strong Lewis acid. Nitrogen participates in the formation of bonds through valence electrons. These are the 3. Nitrogen has 5 valence electrons.
Nitrogen has 5 electrons in its n2 outer shell. To determine valence electrons add the outermost and also orbitals. Is B I just did USA TEST PREP Making a 5050 solution of water and. Nitrogen has 5 valence electrons because its electron configuration is.
For double digit families you just take the second digit and that is the number of valence electrons. Nitrogen is the 7th element so out of 7 electrons 2 go to the first shell and 5 to the second. Nitrogen has 5 valence electrons. Nitrogen is a nonmetal found in group 15 of the periodic table.
What is a period. To determine valence electrons add the outermost s and p orbitals. Nitrogen is in Group 5 so it has 5 outer shell electrons. For the elements that correspond to this group the configuration of their valence electrons is where n corresponds to the period where it is located.
The simple logic is that 5 3 8. Nitrogen is the 7th element so out of 7 electrons 2 go to the first shell and 5 to the second. The number of electrons in the highest energy. However since nitrogen is in the 15 group of family it does not have 15 valence electrons.
How many valence electrons does Silver Ag have. How many electrons should Nitrogen group 15 have around its Lewis dot model. Add your answer and earn points. How many valence electrons does Nitrogen have.
Nitrogen symbol N is found in column 5 on the periodic table. The total number of electrons present in the valence shell of an atom are called valence electrons and there are a total of five electrons present in the valence shell of nitrogen 2s22p3. How many valence electrons does Sulfur S have. The atomic number of Bromine is 35 which means it has 7.

How Many Valence Electrons Does Nitrogen N Have

What Are Valence Electrons Chemtalk

What Is The Number Of Valence Electrons In Nitrogen Socratic

How To Find The Valence Electrons For Nitrogen N Youtube

Periodic Table With Valence Electrons Labeled 7 Hd Images

Nitrogen Definition Symbol Uses Properties Atomic Number Facts Nitrogen Behavior Kindergarten Skills
Komentar
Posting Komentar